Counting atoms in simple molecules with coefficients answer key – Delving into the intricate world of chemistry, we present a comprehensive exploration of counting atoms in simple molecules with coefficients. This fundamental concept unveils the secrets of molecular composition, providing a crucial foundation for understanding chemical reactions and properties.
Throughout this guide, we will unravel the mysteries of counting atoms, equipping you with the tools and techniques to navigate the molecular landscape with confidence.
Counting Atoms in Simple Molecules with Coefficients
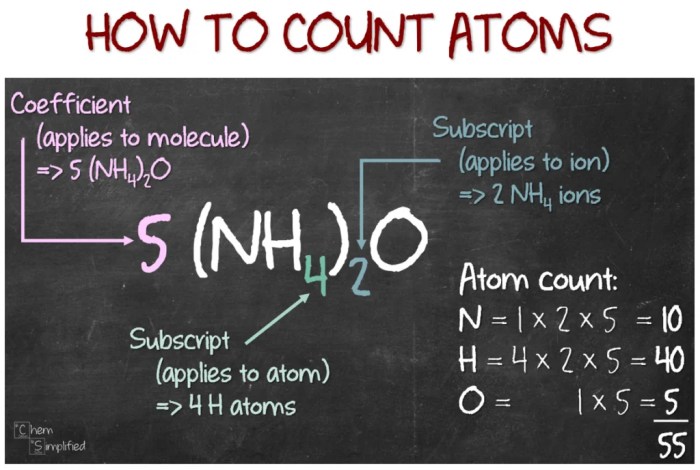
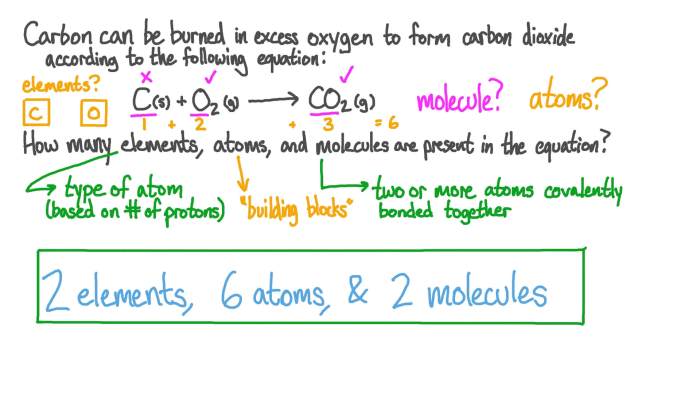
Counting atoms in simple molecules with coefficients is a fundamental skill in chemistry. It involves determining the number of atoms of each element in a molecule, taking into account the coefficients that appear in the chemical formula. Coefficients are numerical factors placed in front of chemical formulas to balance chemical equations.
They indicate the relative number of molecules or atoms of a particular substance involved in a reaction.
Methods for Counting Atoms
To count atoms in simple molecules with coefficients, follow these steps:
- Identify the chemical formula of the molecule.
- Determine the coefficient in front of the formula. If no coefficient is present, assume it is 1.
- Multiply the number of atoms of each element in the formula by the coefficient.
For example, to count the atoms in the molecule 2H 2O, we multiply the number of atoms of each element in the formula by the coefficient 2:
- 2 x 2 = 4 hydrogen atoms
- 2 x 1 = 2 oxygen atoms
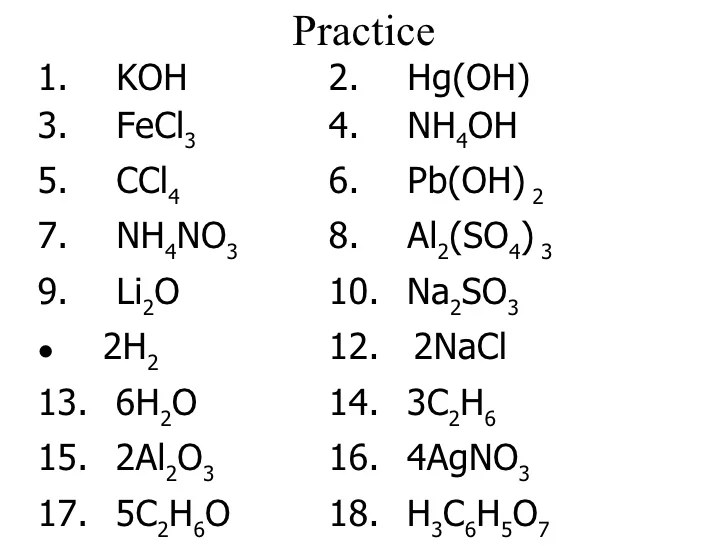
Practice Problems, Counting atoms in simple molecules with coefficients answer key
1. Count the atoms in the molecule CH 4.
Solution:
- 1 carbon atom
- 4 hydrogen atoms
2. Count the atoms in the molecule 3NH 3.
Solution:
- 3 nitrogen atoms
- 9 hydrogen atoms
Applications of Counting Atoms
Counting atoms in simple molecules with coefficients has numerous applications in chemistry and other fields:
- Balancing chemical equations
- Determining the molar mass of compounds
- Calculating the concentration of solutions
- Understanding stoichiometry and chemical reactions
Top FAQs: Counting Atoms In Simple Molecules With Coefficients Answer Key
What is the significance of counting atoms in simple molecules?
Counting atoms provides insights into the molecular structure and composition, allowing us to determine the number of atoms of each element present.
How do coefficients affect the counting of atoms in molecules?
Coefficients in chemical formulas indicate the relative number of molecules or atoms involved in a reaction, influencing the total count of atoms.
What are the applications of counting atoms in simple molecules?
Counting atoms finds applications in various fields, including chemistry, biochemistry, and materials science, enabling the determination of molecular weights, stoichiometry, and chemical properties.